
The Laboratory of Cells and Tissues 3D Imaging and Correlative Microscopy was established in 2020 by modernization of laboratories of light microscopy and transmission electron microscopy. During the investment process, the Laboratory was equipped with the most advanced, often unique devices that allow research using various modern morphological, such as 3D imaging of cells and tissues ultrastructure and correlative imaging. Moreover, the laboratory enables high efficiency routine histological and ultrastructural examinations with full digital documentation of the results. The employees of the Department of Histology and Embryology have years of successful experience in light and electron microscopy research.
 |
Field-emission scanning electron microscope ZEISS GeminiSEM 450 |
High-resolution scanning electron microscope with thermal field emission, double-condenser lens system, beam deceleration and ATLAS 5 equipped with:inside column detectors of secondary and backscattered electrons,extra-column detectors of secondary electron: SE2 and VPSE, retractable detectors of backscattered electrons: Zeiss BSD4, Zeiss SenseBSD and Gatan OnPoint, retractable STEM detector.The microscope is linked to Carl Zeiss ATLAS system and correlative imaging software.
Research opportunities
The microscope enables imaging of the surface morphology of samples, the ultrastructure of cells and tissues by detecting backscattered electrons (from sections placed on a silicon wafer or other substrate) and the ultrastructure of cells and tissues with transmitted electrons (STEM). It allows imaging of large areas of the sample, array tomography and correlation imaging.
 |
Sample cutting and imaging system GATAN 3View 2XP for use in scanning electron microscope ZEISS GeminiSEM 450 |
A system for removing subsequent layers of the sample using a diamond knife and serial ultrastructural imaging in Zeiss GeminiSEM 450 scanning electron microscope. It enables to remove layers of material with a thickness from 30 nm and create single images with a size of 32 000 x 24 000 pixels. The system works with a Gatan OnPoint detector with very high performance and imaging quality. It is equipped with nitrogen injection system on the sample surface.
Research opportunities
The system allows for high-resolution 3D imaging of cells and tissues ultrastructure using the "Serial Block Face Imaging" method, i.e. by cutting the sample in the scanning electron microscope chamber and imaging successively exposed layers. The charge compensation by injecting small amounts of nitrogen allows imaging of samples with low electrical conductivity, e.g. samples with a large amount of voids filled with resin.
 |
Transmission electron microscope FEI Tecnai 12 Biotwin |
A microscope with a maximum accelerating voltage of 120 kV, with a LaB cathode, with a special Biotwin lens for increased contrast, coupled with an Eagle 4k x 4k digital camera from FEI and a Veleta 2k x 2k digital camera from Olympus, equipped with a TEM 3D tomography system.
Research opportunities
The microscope enables the observation of ultrathin preparations at a magnification of 1 500 -300 000x using a fluorescent screen and using the live mode, a Veleta digital camera mounted in the side port, recording images with a resolution of up to 16 million pixels (Eagle camera detector area 6 cm x 6 cm), and acquisition of 3D images from ultrathin sections using the tomography technique.
 |
Microscope slide scanner 3DHistech PANNORAMIC 250 Flash III |
Automatic scanner with the possibility of simultaneous loading of 250 standard histological slides, 20x and 40x objectives, equipped with the xenon flash field illuminator for bright filed imaging and the solid state light source with a spectrum range from 380 to 680 nm for fluorescence imaging, filters for DAPI, FITC, TRITC, Texas Red, mCherry dyes, and Cy5, and.
Research opportunities
The scanner enables the digitisation of microscope preparations in transmitted light and fluorescence, using 20x and 40x magnification objectives. The pixel size in the scan is less than 0.2 micrometres. Multi-channel recording is possible in fluorescence. The scanner allows to collect image stacks from the specimen and convert them into an enhanced focus image. Image viewing software (no license required) allows to perform basic morphometric measurements (length, perimeter, area) and save scan fragments in publication quality.
 |
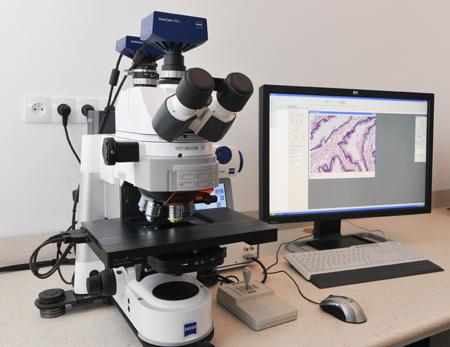
Confocal microscope Zeiss LSM 900 with Airyscan 2 detector |
A system based on a motorised inverted microscope with an incubator, equipped with four lasers with wavelengths of 405 nm, 488 nm, 561 nm and 640 nm, two GaAsP detectors, a high-resolution Airyscan 2 detector, a transmitted light detector, a monochrome camera with a resolution of 6 million pixels and correlation microscopy holders.
Research opportunities
The system enables confocal imaging with super-resolution detection (120-140 nm), image registration using wide-field fluorescence and differential-phase contrast, automatic acquisition of images of serial sections, correlation of examined structures at the level of optical and scanning electron microscopes, incubation of cells and tissues under controlled conditions of temperature, carbon dioxide concentration and humidity.
 |
Motorized Optical Microscope Zeiss Axio Imager with Apotome |
The microscope is equipped with the Axiocam HRm cooled monochrome camera, the MRc5 cooled color camera and the Apotome fluorescent structured lighting system.
Research opportunities
The microscope configuration enables: 1) viewing and image recording of samples in bright field, 2) viewing and image recording of samples with the use of Nomarski contrast, 3) viewing and image recording of samples using the classical epifluorescence technique and using the Apotome system, 4) registration of the "image stack" of collected layer by layer in the Z-axis, 5) registration of large areas of the preparation, 6) obtaining images with a large depth of field, 7) deconvolution of fluorescent images, 8) 3D reconstruction of fluorescent images.
 |
Microscope Zeiss Axio Zoom.V16 |
The microscope is equipped with an apochromatic mono-zoom optical system with automatic change of head magnification in the range of 0.7-11x, 1x and 2.3x objectives, automatic stage movement in the X-axis, automatic movement in the Z-axis, transmitted and reflected light illuminator, epifluorescence illuminator and a color digital camera with 6 million pixels resolution.
Research opportunities
The microscope enables imaging of the surface of spatial objects with an increased depth of field, imaging using the epifluorescence technique, and automatic registration of silicon wafers intended for SEM research. The software allows to correlate images between Axio Zoom.V16 optical microscope and SEMGemini 450 scanning electron microscope.
Software: Explore 3D i TIA (FEI), Axiovison 4.8 (Zeiss), ZEN 3.7 (Zeiss), ATLAS 5 (Zeiss), GMS 3.0 (Gatan), Dragonfly 2020.2 (ORS), Amira 4.0 (Visage Imaging), Amiara 3D 2022 (Thermofisher) Photoshop CS 4 oraz CS5 (Adobe), MIB (University of Helsinki).
A carousel tissue processor with a vapour extraction system enables dehydration and paraffin infiltration of samples for histological and histochemical studies. The device is controlled by a microprocessor and contains 10 stations for chemical reagents and 2 stations for molten paraffin. When one of the nine programs is selected, the processor ensures automatic handling of up to 70 cassettes with preparations simultaneously.
The station consists of two modules: heating and cooling. The heating module is a paraffin distributor used to melt solid paraffin, maintain liquid paraffin at the proper temperature, and pour paraffin into moulds with samples. Additionally, it allows the storage of sample cassettes and moulds at constant temperature. A cooling module containing 60 moulds was used to cool the material embedded in paraffin.
 |
Stainer Integrated Workstation: Leica ST5020-CV5030 |
The integrated system consisting of a stainer and a coverslipper provides automatic staining of histological slides using various methods and coverslips finished slides. The staining process begins by placing the baskets with slides in the loading station of the stainer. The device then performs thermal or chemical removal of the paraffin, rehydration of the sections in a series of solutions of ethyl alcohol of decreasing concentration, staining, dehydration in a series of solutions of alcohol of increasing concentration, and placing in an intermediate fluid. From the last programmed in the staining protocol station, the slide holder removes and transfers slides to the loading station of the automatic coverslipper. Then, a mounting medium is applied to the slide, a coverslip is placed, and the completed slide is put aside to dry. The device allows to save 50 different staining programs.
A fully automatic microtome with a vibrating blade is designed for sectioning fixed and unfixed samples. The device operates in automatic or semiautomatic cutting mode. The range of cutting thickness is 1-20,000 μm with the possibility of variable cutting speed between 0.01-1.5 mm/s. Separate panel with an LED display is dedicated to control the device operation. The vibratome is equipped with trays for buffer and ice. The set includes a magnifying glass (2x) and a Leica CLS 100X illuminator.
A system for freezing biological samples in liquid nitrogen at very high pressure eliminates the formation of significant structural and chemical changes. Chemically unfixed samples can be frozen (then, high-pressure freezing is the only method of their fixation) and samples that are chemically pre-fixed. The sample thickness should not exceed 0.2 mm. The freezing process is fully automatic within one second and after its completion the sample is placed in a container with liquid nitrogen. Freezing parameters are stored in the device memory.
The processor enables automatic or manual fixation with osmium tetroxide, contrast, and dehydration of frozen biological material under programmed temperature changes. The processor also allows embedding the material in the resin and polymerising under low temperature using UV light.
The incubator with controlled evaporation and temperature maintenance system ensures in situ hybridisation on paraffin or frozen sections. The incubator is equipped with a HybEZ™ II Oven, a HybEZ tray for slide incubation with a humidity control lid, an EZ-Batch holder for placing slides in the tray, and an EZ-Batch tray for washing slides.
The device enables coating with non-oxidizing noble elements (gold, platinum, silver, palladium, copper, nickel), oxidising metals (chromium, tungsten, aluminum, titanium, iron, cobalt, tin, molybdenum, iridium, magnesium, tantalum) and carbon (carbon braid or carbon rods), as well as the evaporation of metals and carbon. The coater is characterised by a very fine grain of the coated layer (grain of approximately 0.5 nm). A wide range of turntables ensures uniform coverage of the samples with the guide. The device has a film thickness monitoring system (Film Thickness Monitor, FTM), i.e., it indicates the thickness of the sputtered layer or automatically ends the process after reaching the desired layer thickness. In addition, it is equipped with a plasma surface preparation head (hydrophobic or hydrophilic conversion). The device is fully automatic with possibility of programming the work using the touch screen. The pre-rotary pump and turbomolecular pump are responsible for generating the vacuum in the chamber.
The dryer uses the phenomenon of coexistence of the liquid and gas phases at the critical point, which allows the removal of liquid from the sample (drying) without its deformation. The process is fully automatic which ensures repeatability of results and minimizes the time necessary to prepare the sample.
 |
Coater Quorum Q150T ES and Critical point dryer Tousimis 931 |
The device enables efficient and safe vacuum drying of non-flammeable solvents in the temperature range of 15-200 °C. The APT.lineTM technology allows for condensation-free and homogeneous drying of samples and the Cross-Flow technology ensures fast and full convection. The dryer is equipped with a control panel with time segment programming and pressure regulation.
The semiautomatic manual rotary microtome enables cutting biological material samples embedded in paraffin sections used for the preparation of histological slides.The precision-cutting thickness range is 0.5 ̶ 100 µm. The trim function with defined steps between 5 ̶ 500 µm simplifies accurate distance adjustment and achieves thicker sections. The device has the function of withdrawing the sample at the end of the section to protect the sample and the knife. Additionally, the knife holder is equipped with a cover for the cutting edge. The microtome is equipped with a Cool-Cut cooling device with a clamp for paraffin blocks. Cooling the preparation improves the cutting process, thus improving the quality of the collected scraps.
The manual rotary microtome enables sectioning of paraffin-embedded samples to prepare high-quality histological slides for staining and further analysis under a light microscope. The precision-cutting thickness range is 0.5-60 µm, and trimming is possible in thicknesses of 10 µm and 50 µm. The microtome has a specimen retraction function that protects the knife and probe. The knife holder is equipped with a cover to protect the cutting edge. Additionally, the microtome is endowed in an electrically cooled clamp for paraffin blocks (Leica RM Cool Clamp).
 |
Cryostat Thermo Scientific CryoStar NX50 |
The cryostat enables freezing of the biological samples and preparation of frozen sections for histochemical and immunohistochemical staining. The device is equipped with a stainless steel-cooling chamber with active cooling of the knife and the head with the held sample. Inside the chamber, there is a manual microtome with the possibility of spatial adjustment of the position of the head with the sample. The thickness range of precise section cutting is 0.5-100 µm and the trimming thickness is up to 500 µm. The temperature control range of the cryostat chamber is up to -25 °C, the knife holder up to -25 °C, and the head with the sample up to -45 °C. The device is equipped with a freezer shelf for 18 slides and one quick-freeze position reaching -57 °C. Moreover, the cryostat has the Vacutome function, which simplifies scrap straightening and keeps the chamber clean. The control panel is equipped with a touch screen with intuitive multiuser software.
The device is used for block cutting using glass and diamond knives. The precise manipulation is ensured by a stereoscopic microscope installed in the device. The ultramicrotome is equipped with a control panel with the option of setting the cutting speed and thickness of the slice. It has an automatic block feed with a stepper motor, a 360-degree rotating knife block, knife inclination angle adjustment, and t sample lighting system. The device is placed on an antivibration table, which simplifies the cutting process.
Research opportunities
The ultramicrotome enables perfect preparation of semi-thin (0.5 - 1 μm) and ultra-thin (50 - 80 nm) sections collected on slides or metal meshes, respectively. Scraps are collected manually. The availability of various handles enables cutting blocks of various shapes and sizes. The device also allows trimming, i.e. limiting the surface of the specimen for imaging.
 |
Automated Tape Collecting Ultramicrotome ATUMtome RMC Boeckeler Instruments |
The ultramicrotome is equipped with a unique system for automatically collecting serial ultrathin sections on a special tape (Array Tomography method). It also has an additional holder for placing sections on silicon wafers and slides. The device is equipped with an automatic block feed with a stepper motor, a 360° rotating knife block, knife inclination angle adjustment, and a sample LED lighting system (top, spot, bottom and passing with adjustable intensity). The ultramicrotome is placed on a pneumatic antivibration table and equipped with a camera that allows the cutting to be controlled on a computer monitor.
Research opportunities
The ultramicrotome, in addition to traditional preparations for examination in transmission electron microscope placed on metal grids, enables the preparation of sections on silicon wafers, coverslips and tape. In the case of a tape, it is possible to collect up to several thousand scraps. Sections prepared on silicon wafers, slides, and tape are intended for imaging the ultrastructure of cells and tissues in a scanning microscope. They can be analyzed multiple times with different parameters e.g. in different resolutions. Sections of this type allow imaging of huge areas of preparations, preparation of spatial reconstructions using the Array Tomography technique, and correlation of images in the light and electron microscope. Placing the sections directly on the silicon wafer ensures very high quality and resolution of imaging (even below 1 nm/pixel).
The device is equipped with a precise system for positioning a special glass bar and breaking it into knives with straight edges. The breaker has a pressure sensor and a digital display to control the process and determine the moment of glass breaking. The breaker allows the use of an ultra-microtome to obtain sharp glass knives for trimming synthetic resin blocks and for making semi-thin sections, i.e., with a thickness of 0.5-1.5 μm. Additionally, the glass knife is preferred for cutting the first ultrathin scraps to protect the diamond knife edge from damage.
 |
Beckman LS 6500 scintillation countePerkin-Elmer Tri-Carb 2810 TR scintillation counter, |
 |
Beckman J-6 MC type centrifuge,Beckman Allegra 64R type centrifuge |
 |
Dionex HPLC system with electrochemical detector Coularray 5600Dionex HPLC Ultimate 3000 system with a fluorescence detector |
 |
Zeiss Axiovert inverted fluorescence microscope |
The microscope configuration allows: a) imaging cells in a monolayer culture using phase contrast, PlasDIC contrast and Varel contrast, b) imaging cells using epifluorescence, c) long-term imaging of living cells in an incubator placed on a stage, d) analysis of changes in calcium ion concentration in cells, e) performing microinjections.
 |
Kendro BB6060 incubator with controlled CO2 and O2 levels Galaxy 48R incubator with controlled CO2 and O2 levels |